Abstract
INTRODUCTION
In vivo T-cell depletion with Anti-T-lymphocyte globulin (ATLG; Grafalon) is one of the strategies used in the graft-versus-host disease (GVHD) prevention in the setting of hematopoietic stem cell transplantation (HSCT). One open-label randomized trial in HLA-matched unrelated myeloablative HSCT have reported the reduction of cGVHD with the incorporation of ATLG (60mg/Kg), however progression-free survival (PFS) and overall survival (OS) were lower than in the placebo group1. Other randomized trial in related myeloablative HSCT, using an inferior dose of ATLG (30mg/Kg), have reported this reduction in GVHD results without increasing transplantation-related mortality (TRM) or disease relapse (DR)2.
Based on this, the policy in our center is to use a lower dose of ATLG (21mg/Kg) in unrelated myeloablative HSCT. The aim of this study is to analyze the outcome of this strategy in terms of GVHD results, PFS, OS, TRM and DR.
METHODS
We conducted a retrospective study in all patients who underwent a HLA-matched unrelated myeloablative HSCT at our institution between 2013 and 2021. Patients received a total dose of 21mg/Kg of ATLG divided into three doses of 7mg/Kg on days -3, -2 and -1. The primary endpoints were cumulative incidence (CI) of aGVHD, CI of moderate-severe cGVHD, PFS and OS. Secondary endpoints were TRM, CI of DR and toxicity.
RESULTS
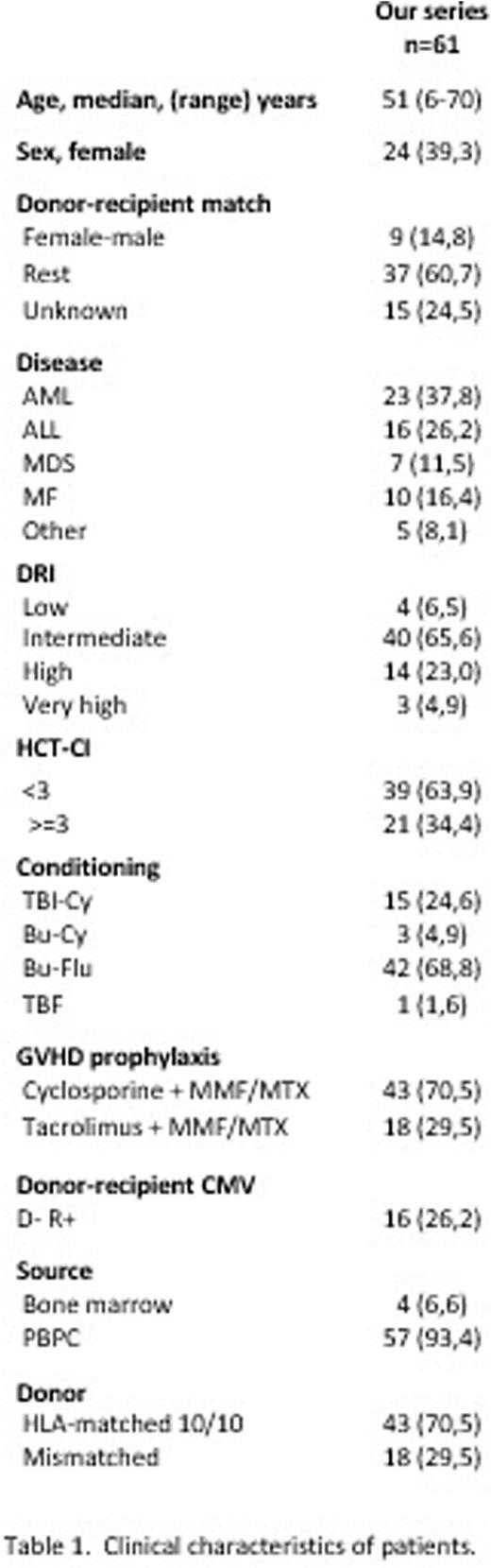
Sixty-one patients were included. The median age was 51 years. Thirty-seven (60,7%) were men. Twenty-three (37,7%) patients had an acute myeloid leukemia (AML). Eighteen (29,5%) patients had an HLA-mismatched donor. Forty-two (68,8%) receive a Bu-Flu conditioning. Cyclosporine combined with mycophenolate mofetil (MMF) or short course of methotrexate (MTX) as GCVH prophylaxis was used in 43 patients (70,5%). Clinical characteristics of patients are showed in table 1. The median follow-up of the alive patients was 36 months.
A median value of 0.1 x 106/L lymphocytes was observed on the first day of infusion. In 35 patients (57,4%) a grade 1 to 2 infusion reaction was reported and in 3 (4,9%) a grade 3 to 4. Those three patients did not complete all infusions, the rest of patients did.
Median time to neutrophil engraftment (3 consecutive days of >500/μL) was 15 days (IR, 13-18) and to platelet engraftment (3 consecutive days of self-supported platelets >20000/μL) was 17 days (IR, 15-23).
CI of aGVHD grade 2 to 4 within 180 days after HSCT was 39,5% (95% CI, 27,1% to 51,6%) and CI of aGVHD grade 3 to 4 was 11,6% (95% CI, 5,0% to 21%), CI of moderate-severe cGVHD at two years was 20,9% (95% CI, 11,4% to 32,4%). PFS at two years was 59,6% (95% CI, 47% to 71%) and OS at two years was 71,8% (95% CI, 59% to 83%). The cGVHD relapse free survival (cGRFS) at two years was 36,2% (95 CI, 24% to 48%). The CI of RD at two years was 23,3% (95% CI, 13% to 35,3%) and the TRM at two years was 17,1% (95% CI, 8,7% to 27,9%).
Median time to cyclosporine or tacrolimus withdrawal was 6,7 months (interquartile range, 5-8 months) and median of use of any type of immunosuppressors (i.e corticosteroids) from the day of HSCT was 6,9 months (interquartile range, 5-10 months).
In 4 (6,6%) patients a primary graft failure was reported, and two patients developed post-transplantation lymphoproliferative disease.
CONCLUSSIONS
The use of ATLG at dose of 21 mg/Kg in HLA-matched unrelated myeloablative HSCT offers promising results of OS and PFS with a low TRM, despite being related to a higher incidence of aGVHD and cGVHD than previously trials were the ATLG dose was higher. It showed a better profile of tolerability and adverse effects. More investigation should be undertaken to determine the appropriate dose of ATLG in this type of HSCT.
REFERENCES 1. Soiffer RJ, Kim HT, McGuirk J, Horwitz ME et al. Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017 Dec 20;35(36):4003-4011. doi: 10.1200/JCO.2017.75.8177. Epub 2017 Oct 17. PMID: 29040031; PMCID: PMC8462523.
2. Kröger N, Solano C, Wolschke Cet al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med. 2016 Jan 7;374(1):43-53. doi: 10.1056/NEJMoa1506002. PMID: 26735993.
Disclosures
Ocio:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy; Oncopeptides: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Amgen, BMS/Celgene, GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Takeda: Consultancy; GSK: Research Funding; Amgen, BMS/Celgene, GSK, Janssen, Oncopeptides, Pfizer, Sanofi, Takeda: Honoraria; Janssen, Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal